Within a few weeks, BioVariance GmbH established a new lab for PCR diagnostics in the former hospital in Waldsassen in February 2021. Up to 1000 samples per day have been tested for SARS-CoV-2 infection since the establishment of the lab. The unique feature: After the samples have arrived in the lab, the processing and analysis of the samples usually needs less than 3 hours. A fast identification of newly infected people is essential for putting them in quarantine and thus avoiding further infections. In collaboration with the BRK, BioVariance made a great contribution to the rapidly decreasing incidence in the county of Tirschenreuth in spring 2021. But how can BioVariance reach this high pace? And how does PCR diagnostics actually work? These and further information about the workflow in the BioVariance lab can be found below.
qPCR diagnostics in the BioVariance lab
The abbreviation „qPCR“ stands for “quantitative polymerase chain reaction”. SARS-CoV-2 is a so-called RNA virus, that means that its genome consists of RNA and not of DNA like in animals or humans. An organism can be clearly identified by analyzing its genome, which is the main point of the PCR analysis. A pharyngeal sample contains RNA in the form of potential viruses as well as DNA in the form of human mucosal cells. Therefore, the contained RNA is isolated and purified before the actual PCR. This RNA is then converted to DNA using the enzyme reverse transcriptase, because the PCR can only work with DNA. After this process the actual PCR starts: The process being called “amplification”, characteristic segments of the produced DNA are multiplied using the enzyme polymerase. BioVariance targets 3 specific DNA segments, while 2 of them belong to genes found in SARS-CoV-2: The RdRP gene, which exists only in SARS-CoV-2 specifically. And the E gene, which occurs generally in the subgenus Sarbecovirus, whereto also SARS-CoV-2 belongs. The evidence of the RdRP gene alone would be enough to identify a sample as positive, but BioVariance presupposes the detection of both virus genes for the generation of a positive report to avoid wrong-positive results. The third targeted segment, the RNAse P gene, is a human gene, which serves as an internal control factor for checking whether the sample contains enough biological material to avoid wrong-negative results.
Evaluation of the PCR analysis

But how can we check whether the amplification is successfull? Using a special PCR cycler (see Fig. 1), fluorescence markers are embedded into the multiplied DNA sequences during the PCR. Every target sequence is associated with another fluorescence colour. The more DNA is produced by the polymerase, the stronger is the fluorescence of the sample in the real time measurement. For generating an unequivocal result – no matter if negative or positive – the fluorescence signal of the human RNAse P gene must be sufficient. If there is additional fluorescence of both virus genes, the sample will be marked positive. The Ct value serves as a scale unit for the number of existing genes. It describes the number of DNA multiplication steps being necessary to differentiate the fluorescence signal clearly from the so-called background noise and to generate a reliable test signal. The lower the Ct value is, the higher is the number of existing target genes and thus the number of virus particles. Conversely, a high Ct value shows a low concentration of virus particles. During the PCR, the fluorescence signal arises until it peaks after about 1 hour. After this peak it flattens out because the added components for DNA multiplication are used up. This process creates the typical sigmoidal curve of the fluorescence (see Fig. 2).
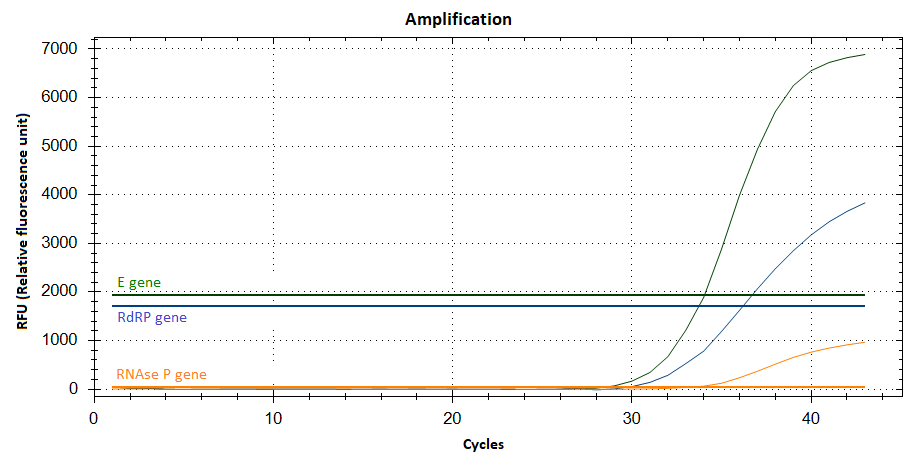
But even the best procedure is subjected to limitations. In about 1 % of cases the result of the PCR is not unequivocal, because the sample material is insufficient or the fluorescence signal is too low, for example. In these cases, the PCR is repeated to gain a clear result.
Identification of virus variants using qPCR
In the BioVariance lab all positive samples additionally undergo a special variant PCR analysis to identify the respective SARS-CoV-2 variant, targeting several SARS-CoV-2 specific gene segments containing the yet known mutations. These analyses revealed that in the last weeks the former virus, the so-called wildtype, was gradually replaced by the British variant B.1.1.7, today called “Alpha“, in the county of Tirschenreuth. Furthermore, positive samples are also sent to a partner lab of BioVariance for further variant analyses, so that new and yet unknown mutations can be identified.
Screening of schools using pooled gargle samples
In May 2021, BioVariance has introduced a new pooling procedure, with several schools in the county of Tirschenreuth participating in the context of a study. This procedure does not need the pupils to take a nose or pharyngeal sample, but only to gargle with some tap water in the morning at home. At school, these gargle samples are “pooled” per class, that means mixed in one sample cup (see Fig. 3). Thus, only one PCR analysis has to be done for each class. Only if a pool sample is positive, the gargle samples of the referring pupils will be analyzed individually. This process allows great savings of lab resources while maintaining a high quality of results.

Quality management in the BioVariance lab
BioVariance regularly faces external evaluations to ensure the quality and safety of their PCR analyses. For these so-called ring trials, the lab receives several unknown samples from a central office. Only if all samples are identified correctly as negative or positive, the lab will pass the trial and gain a certificate. In May 2021, BioVariance has passed again the external test from the European Society for External Quality Assessment (ESfEQA). Further, all incoming samples are treated under sterile conditions and conforming with strict hygienic regulations in the BioVariance lab, avoiding the contamination of the workstations and a possible infection of employees (see Fig. 4).

Speed of PCR diagnostics in the BioVariance lab
In Germany, it usually takes 24-48 hours until a tested person receives the PCR result. In the BioVariance lab, results can be produced in less than 3 hours after the samples arrived in the lab. So 99 % of the tested people receive their result at the same day. Two special components allow BioVariance to reach this speed: On the one hand, BioVariance uses an optimized procedure for the RNA extraction which guarantees high efficiency, precision and safety. On the other hand, the IT specialists of BioVariance developed a purpose-built software called TubeLab enabling the digital monitoring of the lab processes, which clearly accelerated and facilitated the handling of samples. Using TubeLab for a transparent tracing and documentation, the BioVariance lab meets the highest requirements. Besides, the partner lab doing the further sequencing of the positive samples also uses TubeLab for monitoring their PCR analyses since April 2020. BioVariance also increased its personnel and expanded the lab team by 2 technical lab employees and 2 student assistants from the scientific area.
Strategic success in Tirschenreuth
With the help of the administrative district office and the health office, the administrative barriers for the establishment of the diagnostic lab as from 26.02.2021 in Waldsassen could be removed within a very short time. Short ways between the test station and the lab are indispensable for rapid testing. In the best case, both stations are located in the same building like in Waldsassen. And thanks to the BRK, 4 permanent and several mobile test stations could be established. More than 40.000 pharyngeal samples from people living in the county of Tirschenreuth have been analyzed since the commissioning of the lab. Using the highly efficient PCR diagnostics, infected people could be identified and quarantined within a few hours. This fact also had a strong positive influence on the local incidence. In February 2021 when the lab was commissioned, the 7-day-incidence was more than 350 infections per 100.000 inhabitants in the county of Tirschenreuth. Now, 15 weeks later, the region is the first county in Bavaria to show a 7-day-incidence of 0. The effective collaboration of the administrative district office, the health office, the BRK and the BioVariance lab as well as the consistent endurance of the population made it possible to signficantly reduce new infections and to finally conquer the high incidence in the county of Tirschenreuth.
Future orientation of the BioVariance lab
BioVariance aims to expand the application possibilities in the lab and to integrate the diagnostics of cancer diseases into their portfolio, additionally to the existing diagnostics of infectious diseases. The company has always focused on the personalization of cancer diseases by sequencing tumors and using the homemade software OncoVariant. This pioneering step allows the selection of the most suitable therapy for cancer patients individually to achieve the greatest possible effectiveness. Further, BioVariance plans to take advantage of the mass spectrometry, which allows the measurement of metabolic products for example. Conntact person: Kerstin Hammer




