
The blog post this week is about CRISPR/Cas and because of the World-AIDS-Day last Saturday it is also about first successes in HIV treatment with this genome editing tool and about a message that has shocked many people last month.
DNA structure

The blog on Black Friday already concerned about chromosomes and DNA. Just as a quick reminder: DNA is a long, chain-like polymer composed of individual buiding blocks called nucleotides. A nucleotide consists of a phophate, a sugar and one of four bases (adenine (A), cytosine (C), guanine (G) and thymine (T)). The structure was decrypted in 1953 by Watson and Crick.1 They found that the DNA forms a double helix. Two such chain-like polymers, also called single strands, assemble in opposite directions called complementary. The backbone is formed by the phosphate and the sugar, while the bases pair with each other via hydrogen bonds, always adenine with thymine and cytosine with guanine.
CRISPR/Cas – Definition
To come to CRISPR: CRISPR stands for clustered regularly interspaced short palindromic repeats. In this case, a palindrome is a sequence in which the bases on one DNA strand are in the same order as those on the complementary strand – only in the opposite direction.
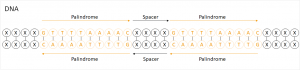
Although such sequences are preceded by a promotor responsible for transcribing DNA into RNA and then translating into proteins, they don’t yield a funtional protein. The point of it wasn’t known for a long time.
Already in 1987 Yoshizumi Ishino discovered the existence of repeating DNA segments in the bacterial strain Escherichia coli K12. He identified similar sequences of each 29 nucleotides interrupted by a sequence of 32 nucleotides, which was variable.2 In 1993 a similar motif was discovered in the DNA of Mycobacterium tuberculosis, one of the vectors of tuberculosis.3 In 2002 similar structures were observed in the genome of many bacteria and archaea belonging to the prokaryotes and the term CRISPR was coined. In addition, a group of genes close to the CRISPR sequence was found in all studied organsims and therefore designated as CRISPR-associated genes (Cas-genes).4 In 2005 it was found out that the sequences located between the palindromic sequences, termed spacers, are identical to the DNA of viruses.5,6,7

According to estimates, there are approximately 4-6×1030 prokaryotes on earth and about ten time more viruses exist. As these organisms depend on host cells for survival, as mentioned in the previous blog they kill about half of the prokaryontes every two days.
Thus it was hypothesized that CRISPR defends bacteria and archaea against foreign DNA of viruses and thus functions as an immune system.5,6,7 In 2007 this claim was confirmed by Barrangov and colleagues, who showed that bacteria infected with phages (groups of viruses) integrated parts of the virus DNA as spacers between the CRISPR regions and thus built up immunity to these pathogens: artificially inserted spacers containing the sequence of viruses made the bacteria resistant to the foreign DNA. The experiment also worked the other way around – the bacteria lost this adaptive restistance when they deleted the spacer regions again.8
So, it has been shown that prokaryotes have an adaptive immune system allowing them to remember DNA sequences from pathogens, similar to humans through antibodies and when they get re-infected, the DNA of the virus can be cut. In detail, the mechanism probably works like this: when it comes to an infection, the DNA of the virus is split into small fragments and inserted into the CRISPR sections. If it comes to a renewed attack, these segments are transcribed into RNA – therefore the upstream promotor – and the viral DNA is checked.If this matches the „stored one“, it is intersected by DNA-cutting proteins, the Cas proteins and thus destroyed.9
Genome Editing

This recognition and cutting of a sequence has been used in so-called Genome Editing for quite some time. Genome Editing is a precise tool that can be used to selectively alter DNA. Genes can thus be inserted into an existing sequence, be removed or be completely replaced. Even individual nucleotides can thus be changed.
A new era in molecular biology was the discovery of restriction enzymes in the 1970s: these enzymes recognize and cleave characteristic sequences.10 The problem here is that the target site is difficult to predict because the recognition sequence is usually only a few base pairs long and thus may occure more frequently in the genome.
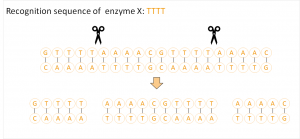
CRISPR/Cas largely prevents such unwanted effects. In addition, it is relaible, fast and in cheap. The principle, like other Genome Editing methods, is based on the three following steps: first the site which should be changed in a targeted way has to be found in the genome. A probe or a vector is constructed containing the desired target sequence, also referred to as gRNA (guideRNA) and the sequence of the molecular scissors – the protein Cas9. Once arrived at the target sequence, Cas9 can cut the DNA double strand exactly at the desired site. Finally, the cell’s own repair system comes into play and rejoins the severed DNA strands.11 Here, the cell has two possibilites: non-homologous end-joining (NHEJ) or homologous recombination (homology directed repair, HDR).
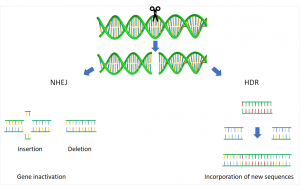
With NHEJ, random insertions or deletions of single nucleotides result in gene inactivation because the reading frame (ORF; the region encoding a potential protein) is thereby shifted. With HDR, a template is added to the cell to give the repair system the information what new sequences are to incorporate.
Gene therapy

So far, patients with defective genes have been treated by a classical gene therapy. They were treated with a virus carrying the sequence of the new functional gene and incorporates it into the affected cells. But also here the problem is that you can’t predict exactly where the gene is integrate in the genome.12 With CRISPR this circumstance is avoided and the target site is exactly predetermined.
CRISPR/Cas in HIV therapy
There have been initial successes in the treatment with CRISPR/Cas, for example in HIV therapy. Although the infection with the HI virus is largely under control by retroviral therapy (ART), a complete cure doesn’t exist yet. Experiments have shown that CRISPR/Cas can effectively fight against HI viruses. Using the molecular scissors, the team led by Kamel Khalili and Won-Bin Young has removed viral DNA from more than 95% of infected cells in mice as well as in human immune cells transferred to humanized mice.13
However, there is still no prospect of an application on humans. Because even this method isn’t without risks. On the one hand, only 95% of the infected cells are removed, the rest is enough that the disease is breaking out again. On the other hand, a virus is used to transport the CRISPR DNA triggering an immune response in the human body and thus can only be used once. In addtion, the transport viruses are suspected to trigger tumors.14
This year, another working group has destroyed the regulatory genes of HIV by CRISPR/Cas so the virus can’t replicate in infected cells. They designed a vector expressing Cas9 and one of six different gRNAs. These vectors were transfected into cells expressing the regulatory proteins of the virus (Tat and Rev). As a result, they were able to record a significant reduction in the expression and funtion of the proteins without affecting the survival of the cells.15 These two publications show that CRISPR/Cas actually would be able to cure HIV-infected people.
Last month a message from China shocked many people. A scientist claims to have helped make the world’s first genome-edited babies. He Jiankui fertilized a woman with embroys, in which CRISPR/Cas was used to destroy the CCR5 gene. This gene encodes a receptor protein, HIV is able to attach in the case of infection and thus is esential for the virus. The aim of the work was not to prevent the transmission of the virus by the parents, but rather that the children don’t have to suffer the same fate as their parent and are thus resistant to the virus.16
Criticism came from all sides. An important argument is that there are also some HIV strains using another receptor protein (CXCR4) for infection.15 In addition, there is the ethical aspect. In Germany, USA and many other countries such manipulations are prohibited. The changes brought into the genome due to Genome Editing will also be passed on to subsequent generations, so the associated risks are scarcely to be estimate.17 Therefore, the use of this silver bullet remains to be enjoyed with great care.
Contact Person:
Kristina Schraml (kristina.schraml@biovariance.com)



