The problem
Generally, drugs are only allowed to be prescribed when special agencies have tested them on safety, effectiveness and quality and have approved them on corresponding indications. The prescription of drugs without considering these facts is called “off-label use”. [1] The relevant aspects for a drug approval are not only the application area and route of administration, but also dosage, moment of intake and the duration of the treatment. Off-label use is not permitted officially, but requires special care. General risks like decreased effectiveness or side effects are harder to conceive due to a lack of study data on effectiveness and safety. [1,2] In new-born, off-label use concerns up to 90 % of all patients, while it’s about 50 % in children and about 25 % in adults. [3,4] Insurances may take over the accruing costs only in these particular cases: [1]
- Severe or life-threatening diseases
- Reasonable assumption that the prescribed drug will induce a therapy success
- Other therapy options are not available
Off-label use in children and adolescents
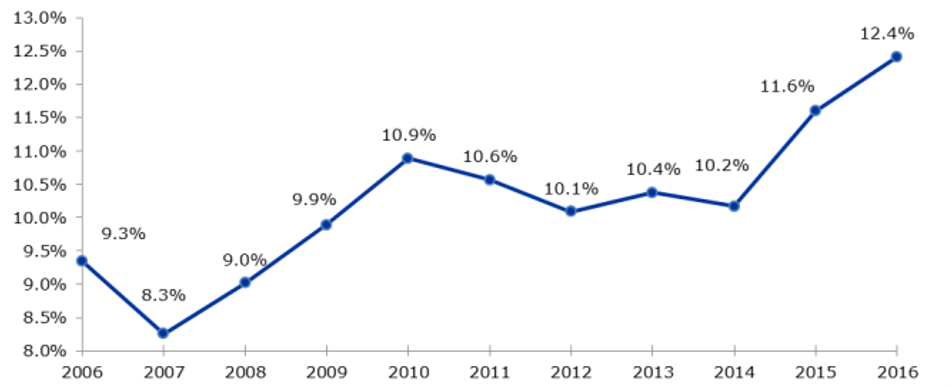
Off-label use is common especially in paediatrics, because clinical studies regarding drug intake in young patients are often lacking. Children and adolescents differ in body size and functions from each other and from adults. Thus, drugs which are originally approved for adults can cause overdosage or severe side effects in children. [2,5] But despite that, ill children have to be treated sufficiently and so the treating physicians are forced to act in this legal grey area. When children are included in clinical studies, there have to be considered special ethical and legal facts. For the participation in a clinical study the consent of the participant (children with an age of 8 years, dependent on development) and his legal representative has to be recorded. In contrast to adult studies, a remuneration is not allowed in studies with children. Further, single indications will probably never get a full approval in rare diseases, because the number of patients in relevant age groups is too low to conduct clinical studies. [5] To improve the current situation of healthcare for children and adolescents regarding approved drugs, the European Union implemented the regulation (EC) Nr. 1901/2006 in the year 2007. The aim was to maintain and extend the number of drugs approved for the treatment of young patients under 18 years. [6] A Pediatric Commission (PDCO) was established for the valuation of paediatric concepts of drug companies. Since the implementation of the regulation clinical studies were increasingly conducted, about 100 new indications were authorized and more than 270 new drugs were approved for the treatment of children (see Fig. 1). [2,7]
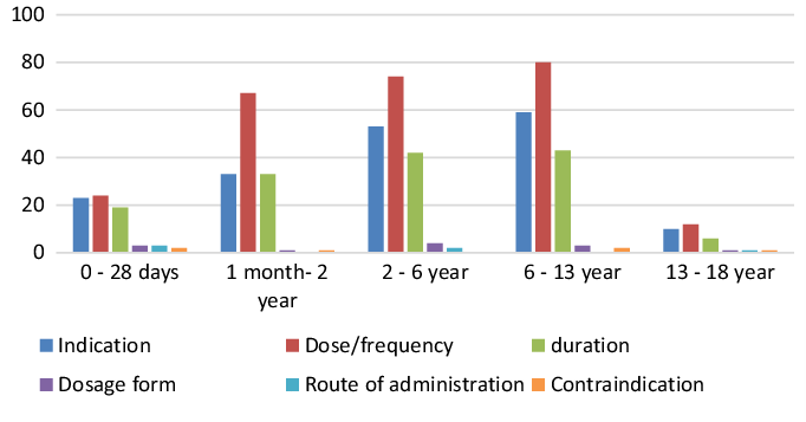
In the year 2016, most of the off-label prescriptions were recognized for patients between 1 and 13 years. The highest number was listed for patients in the age group 6-13 years. Off-label use in dosage, indication and duration of the treatment were highest among all patients (see Fig. 2). [8]
Advancement through digitalization
With the paediatric off-label use being necessary way too often, several countries implemented special projects dealing with the development of web-based platforms. These platforms are meant to support physicians in treating their young patients. Well-known examples are „Kinderformularium“ from the Netherlands, the „British National Formulary for Children“ from Great Britain, „Kispiportal“ from Switzerland and „Drugbase“ from Germany. [9,10,11,12]
- „Kinderformularium“ is an open access paediatric database for physicians and pharmacists. It currently includes information about dosage, application form, side effects, contraindications and corresponding references for more than 730 drugs. [9]
- The „British National Formulary for Children“ was established for health professionals by the Royal College of Paediatrics and Child Health (RCPCH) and the Neonatal and Paediatric Pharmacists Group (NPPG). It deals with prescriptions and care for young patients and offers information about more than 900 drugs and 200 treatment options. [10]
- „Kispiportal“ is the official database of the Universitäts-Kinderspital in Zurich. After entering patient’s data, the correct dosage of a certain drug is calculated automatically. [11]
- „Drugbase“ offered by Wissenschaftliche Verlagsgesellschaft Stuttgart includes several reference works in the fields of medicine and pharmacy, for example the Red List, drug profiles and paediatric dosage charts. [12]
These comprehensive and easily accessible databases will improve therapies for young patients by avoiding side effects and unnecessary expenses and also by shortening the duration of the treatments.
Contact Person: Kerstin Hammer Sources




