
The last articel this year is about pharmacogenetics and answers, among other things, the question of why some medications don’t have an effect on certain people.
Pharmacogenetics – Defintion

Pharmacogenetics is a relatively new branch of classical pharmacology.1 First, it’s necessary to distinguish between the terms pharmacogenomics and pharmacogenetics: Pharmacogenomics describes the alteration of the gene expression profile in the prescence of drugs, while pharmacogenetics addresses the causes of these changes at the level of individual genetic sequences of an organism.2 Heritable characteristics of pharmacokinetics and pharmacodynamics are investigated with the aim of optimzing drug therapy according to the genetic makeup of the patient. Thus, pharmacogenetics combines methods of clinical pharmacology with those of modern genetics.1
Pharmacogenetics – History
Since the 1950s it has been known that people react differently to medication. The first indication was provided by a muscle relaxant called suxamethionium used for anesthesia.3 It was shown that in 1:3,500 people the period of anesthesia was greatly prolonged, as the for degradation necessary enzyme pseudocholinesterase was present in lower concentration in these people.3 Another example, also from the 1950s, was found in 10% of African Americans fighting in the Korean War. They became anemic after taking a certain antimalarial drug, which was due to a particular isoform of glucose-6-phosphate dehydrogenase.2 Based on various observations in this field, the human geneticist Friedrich Vogel coined the term pharmacogenetics in 1959.2
Personalized Medicine
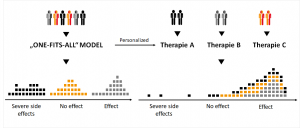 One comes with the personalized medicine. Its aim is to transform the traditional one-fits-all-model into targeted therapies. For this purpose and with the help of modern diagnostics, individual, genetic information is used to find the right drug, in the right dosage, for the right patient at the right time.
One comes with the personalized medicine. Its aim is to transform the traditional one-fits-all-model into targeted therapies. For this purpose and with the help of modern diagnostics, individual, genetic information is used to find the right drug, in the right dosage, for the right patient at the right time.
Why some medications show no effect?
Lack of adherence
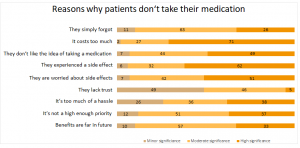
One reason why drugs don’t work is, of course, due to the patient who doesn’t follow the therapy recommenations.4
Side effects and drug interactions
 The main reasons why patients don’t take their medications are the worries about side effects or the fear of experience a new one.4 And this is understandable: In Germany, between 25,000 and 58,000 people die from side effects or drug interaction per year.5 In the United States there are 100,000 deaths per year and around two million patients complaining about unpleasant side effects such as allergies or gastrointstinal problems.6
The main reasons why patients don’t take their medications are the worries about side effects or the fear of experience a new one.4 And this is understandable: In Germany, between 25,000 and 58,000 people die from side effects or drug interaction per year.5 In the United States there are 100,000 deaths per year and around two million patients complaining about unpleasant side effects such as allergies or gastrointstinal problems.6
Lack of evidence base
23% of all Germans take three or more drugs per day, with advanced age (>70 years) the number increases to almost 50%.7 One third of all drugs are prescribed without any evidence base meaning without any scientific evidence for their benefit.5
This also becomes clear in cancer therapy. 57% of the 68 oncology drugs approved between 2009 and 2013 had no real scientific evidence to provide survival benefits or an improvement of quality of life.8 After five years on the market, only 51% had shown therapeutic benefits.8
Responder/Non-Responder
Patients can be devided into two groups: so-called responders showing an effect on a given drug and so-called non-responders showing no effect on a given drug or the intake is associated with side effects.9 An example from cancer therapy makes this clear: 75% of all cancer patients are placed in the second category meaning that these people don’t respond to the prescribed drug.9 Due to the ineffective medication, precious time is lost in which the cancer can spread further.9 The side effects occuring unnecessarily affect the quality of life of patients.9 It also creates huge health system costs that could be avoided.9
Genetics
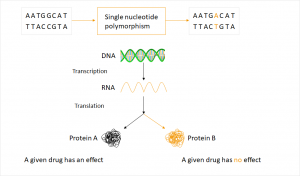
Meanwhile it is known that a significant proportion of the ineffectiveness of drugs is genetically determinded. “There are about seven billion people and each oft hem reacts differently to a drug“ – Dr. Josef Scheiber, Managing Director and Founder of BioVariance GmbH. Living beings metabolize foreign substances with complex enzyme systems that vary from individual to individual. Genes encoding for potential enzymes occur in different variants, which is called polymorphism.  Different variants of a gene are called alleles. The most common sequence variation is the single nucleotide polymorphism (SNP), in which only a single base is different. This variation in the genome is inheritable and doesn’t equate to a mutation describing a newly occurring change.
Different variants of a gene are called alleles. The most common sequence variation is the single nucleotide polymorphism (SNP), in which only a single base is different. This variation in the genome is inheritable and doesn’t equate to a mutation describing a newly occurring change.
Pharmacogenetics – main principle
 The underlying principle of pharmacogenetics is based on these small differences in the genome.1 Approximately every 1,000 nucleotide of the 3.2 billion base pairs of our genome is different.6 This corresponds to around 3 million genetic variants making us unique.6 Relevant for pharmacogenetic consideration is given to polymorphic genes meaning genes with little deviation in the sequence (allele), if they exist with a frequency of >1% in the population and if at least one polymorphism alters the activity of the affected enzyme.2
The underlying principle of pharmacogenetics is based on these small differences in the genome.1 Approximately every 1,000 nucleotide of the 3.2 billion base pairs of our genome is different.6 This corresponds to around 3 million genetic variants making us unique.6 Relevant for pharmacogenetic consideration is given to polymorphic genes meaning genes with little deviation in the sequence (allele), if they exist with a frequency of >1% in the population and if at least one polymorphism alters the activity of the affected enzyme.2
The effectiveness of medications depends on the appropriate mechanism of the drug, the administered dosage and the metabolism of an organism.10 In this case, the liver plays a central role.10 In addition to the production of bile, it also fulfills important funtions in the metabolism of food as well as toxins or drugs (biotransformation).10 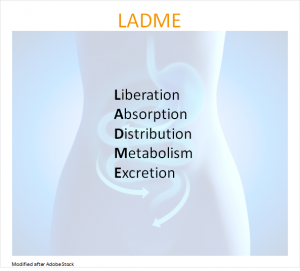 The abbreviation LADME summarizes the processes that take place when taking a drug: Liberation, Absorption, Distribution, Metabolism and Excretion.
The abbreviation LADME summarizes the processes that take place when taking a drug: Liberation, Absorption, Distribution, Metabolism and Excretion.
As an example serves the cytochrome P450 superfamily. It plays the most important role in the metabolism of drugs as monooxygenases in the liver.11 CYP2D6 is involved in the metabolism of approximately 25% of all drugs (especially neuroleptics and antidepressants).1 In the encoding gene, alleles are known due to which the enzyme activity is diminished, completely absent or even increased.1  About 7% of the white population are homozygous carriers of the deficient allele and thus the enzymes have no activity.12 These patients are called poor metabolizers.12 A usual drug dose causes a markedly increased drug reaction, which is toxic in the worst case.12 Tamoxifen plays an important role in breast cancer therapy.13 This is activated via CYP2D6 to the working metabolite endoxifen.13 However, 10% of all patients don’t produce this enzyme, so this drug has no effect.13 Approximately 1,5 – 5% of the population are called ultrafast metabolizer.1 They show a gene duplication, which means that they have three active alleles and thus a significantly increased enzyme activity.1 A common dosage isn’t sufficient in this case.1
About 7% of the white population are homozygous carriers of the deficient allele and thus the enzymes have no activity.12 These patients are called poor metabolizers.12 A usual drug dose causes a markedly increased drug reaction, which is toxic in the worst case.12 Tamoxifen plays an important role in breast cancer therapy.13 This is activated via CYP2D6 to the working metabolite endoxifen.13 However, 10% of all patients don’t produce this enzyme, so this drug has no effect.13 Approximately 1,5 – 5% of the population are called ultrafast metabolizer.1 They show a gene duplication, which means that they have three active alleles and thus a significantly increased enzyme activity.1 A common dosage isn’t sufficient in this case.1
Therapy
Advances in molecular genetics lead to a better understanding of certain diseases and thus to new therapeutic options.2 The aim is to provide a tailor-made therapy for a patient according to his genetic makeup or the genetic characteristics of the illness and thereby to improve the effectiveness and safety.1 Therefore, in addition to non-genetic factors such as environmental factors, food composition, lifestyle, age and body weight and size, also genetic factors accounting for the variability of pharmacokinetic properties and differences in drug efficiency are considered.1 The starting point was the Human Genome Project, which was declared officially as terminated in 2003.14 It was an international research project with the objective of fully decrypting a human genome, so to find out the sequences of 3 billion base pairs in the human DNA.15 The knowledge gained from this project should serve as the basis for investigation of many biological processes, such as heriditary diseases or molecular mechanisms leading to cancer.15
Cancer therapy
In cancer research major advances have been made at molecular level. Cancer isn’t, as previosly assumed, a disease of a single organ, but rather a disease of the genome.16 This is due to changes in one or more genes.16 Thus, every cancer differs from another.16 Humans have around 25,000 genes, of which, according to the current state of knowledge, about 300 are cancer-relevant.16
The negative side of the previous cancer therapy is well knwon. Due to the administration of cytostatics not only cancer cells are destroyed, but also the healthy body cells are attacked leading to serious consequences. In addition, about 51% of the cytostatics, which are used, don’t show the expected success.8 In the fight against cancer there were only surgery, radiation and chemotherapy available until the year 2000, but personalized medicine will open up new possibilities. Cancer is still the second leading cause of death in Germany as well as in the US.17 In addition, the number of new cancer patients is increasing year by year. This year it is estimated, that 493,600 new cases will be added in Germany, worldwide about 18.1 million.18,19 Forecasts predict that this value will raise to 21.6 million by 2030 and to 29.4 million by 2040.20,21
Summary
So far, physicians have opted for a particular therapy based on their knowledge of disease, their experience with medication and patient data. If these don’t work, a different dose or another drug was selected. Personalized medicine now offers the possibility of an idividual treatment. Benefits result for the patient, as well as for the doctors and companies in the health care:
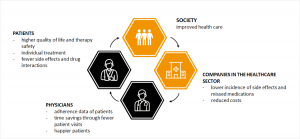
However, not only the ideal therapy is important, but also the possibility of more precise diagnoses. To summarize, the goal of personalized medicine is to find different causes of diseases and to tailor the treatment accordingly to the patient.
Contact Person:
Kristina Schraml (kristina.schraml@biovariance.com)



